A student prepares 100 mL solutions of 01 M HCl 01 M HC 2 H 3 O 2 and 01 M CaCl 2. Copies of both sides of this formula sheet follow the final question.
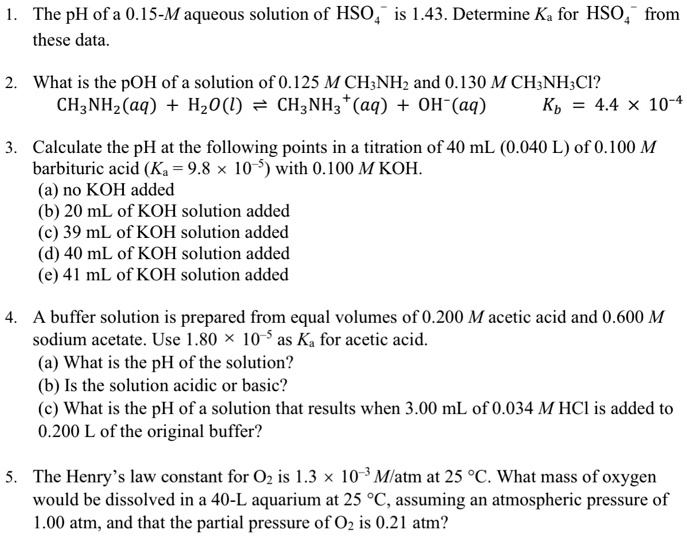
Solved The Ph Ofa 0 15 M Aqueous Solution Of Hso Is 1 43 Determine Ka For Hso From These Data What Is The Poh Of A Solution Of 0 125 M Ch Nhz And 0 130 M Ch Nh Cl
Conducts electricity turns blue litmus to red reacts with Zns to produce gas bubbles Which compound could be the solute in this solution.

. Equal volumes of 01 M NaOH and 01 M HCI are thoroughly mixed. CeO 2 was added and the suspension was stirred at pH7 and at 70 C for 1h. When a 01 M hydrochloric acid solution is tested the bulb glows brightly.
Would you expect the bulb to glow more brightly stop glowing or stay the same as very pure water is added to each of the solutions. 01 mole aqueous solution of an electrolyte A B 3 is 9 0 ionised. 1A student tested a 01 M aqueous solution and made the following observations.
Reacts with Zn s to produce gas bubbles. 48 A student tested a 01 M aqueous solution and made the following observations. Rank the three solutions from highest to lowest vapor pressure.
Answers 1 A student tested a 01 M aqueous solution and made the following observations conducts electricity turns blue litmus to red and reacts with Zn s to produce gas bubbles. A dark coating of copper metal appears on the zinc within two minutes and when 45 minutes have elapsed there is a thick coat of copper metal powder on the zinc strip and the blue color of the solution has lightened. Which compound could be the solute in this solution.
Turns blue litmus to red. The suspension was centrifuged and washed to remove chlorine and sodium ions until the pH reached a steady value. C It turns litmus blue.
Wear your PPE and wash your hands thoroughly. When a 01 M aqueous ammonia solution is tested with a conductivity apparatus see Figure 6-1 the bulb glows dimly. Each student taking the high school Chemistry test was provided with a Chemistry Formula and Constants.
Hydrochloric acid is the aqueous solution of hydrogen chloride gas HCl. 2 B A C 4CBa 12 Which compound releases hydroxide ions in an aqueous. A student is instructed to make 0250 liters of a 0200 M aqueous solution of CaNO32.
Conducts electricity turns blue litmus to red reacts with Zns to produce gas bubbles Which compound could be the solute in this solution. Rank the three solutions from highest to lowest freezing point. HCl solutions are usually prepard by dilution of the concentrated solution.
Turns blue litmus to red. In order to prepare the described solution in the laboratory. Volume of one drop 100010 mL.
As an acid solution is added to neutralize a base solution the OH- concentration of the base solution 1 decrease 2 increases 3 remains the same 13. The compound that could be the solute in this solution is HBr. PART II Reactivity of Aqueous Solutions and Net Ionic Equations Materials.
H 2 SO 4 is a strong acid and very corrosive. B It forms OH ions. 29 A 01-M aqueous solution of _____ will have a pH of 70 at 250eC.
The answer is number 3. The aqueous solution of H 2 PtCl 6 110 -2 M or HAuCl 4 110 -3 M was adjusted to pH of 7 by adding NaOH and heated to 70 C. SheetPeriodic Table of the Elements.
It is a strong irritant to eye and skin and fumes are harmfull if inhaled. 11 The diagrams below show a 01 M aqueous solution of HCl and a 01 M aqueous solution. If 87 grams of K2 SO4 molar mass 174 grams is dissolved in enough water to make 250 milliliters of.
K b H 2 O 0. Ph of an aqueous solution of 01 m nh4cl solution is given pkb nh4oh 47 - Get the answer to this question and access a vast question bank that is tailored for students. 01 M HC 2 H 3 O 2 01 M NH 4 OH 01 M BaOH 2 DI water 50 mL beaker straw conductivity apparatus with light bulbs ceramic well plate Kimwipes sand paper Warning.
When a 01 M hydrochloric acid solution is tested the bulb glows brightly. This volume has been divided into 1000 drops. Total volume V 10 mL 10.
D It turns phenolphthalein pink. How to prepare 01 M hydrochloric acid. NaOCl KCl NH4Cl CaOAc2 ANaOCl BKCl CNH4Cl DCaOAc2 EKCl and NH4Cl 30 An aqueous solution of _____ will produce a basic solution.
Concentrated hydrochloric acid 36-38 is a colorless to yellowish pungent liquid. Solution what are the concentrations of the potassium and the sulfate ions. Would you expect the bulb to glow more brightly stop glowing or stay the same as very pure water is added to each of the solutions.
When a 01 M aqueous ammonia solution is tested with a conductivity apparatus Figure 4-2 the bulb glows dimly. 40 M and 20 M. KCN Ka of HCN 40 x 10-10 b.
Up to 24 cash back 10. H2SO4 aq and Ca OH2 aq A student tested a 01 M aqueous solution and made the following observations. A It conducts electricity.
Rank the three solutions from highest to lowest electrical conductivity. The boiling point of the solution at 1 atm is. 5 2 K kg m o l 1 Medium.
Conducts electricity turns blue litmus to red reacts with Zns to produce gas bubbles Which compound could be the solute in this solution. When zinc metal is immersed in a solution of 01 M aqueous copperII sulfate solution c opper metal plates out on the zincThe solution is initially blue in color. A 10 L sample of an aqueous solution contains 010 mol of.
The property ie remains the same if a solution is divided into n equal parts. Hence the correct option is A. ANaCl BNaHSO4 CKBr DNH4ClO4 ENa2SO4 31 Of the following the acid strength of _____ is the greatest.
The resulting solution has a pH closest to 10 I l. NH4NO3 Kb of NH318 x 10-5. A student tested a 01 M aqueous solution and made the following observations.
Alternative Concentration is intensive. 1 CH3OH 2 LiBr 3 HBr 4 LiOH Base your answers to questions 73 through 75 on the graph below. Of the compounds below a 01 M aqueous solution of _____will have highest pH a.
Which laboratory test result can be used to determine if KCls is an electrolyte. For solution for drops. A student tested a 01 M aqueous solution and made the following observations.
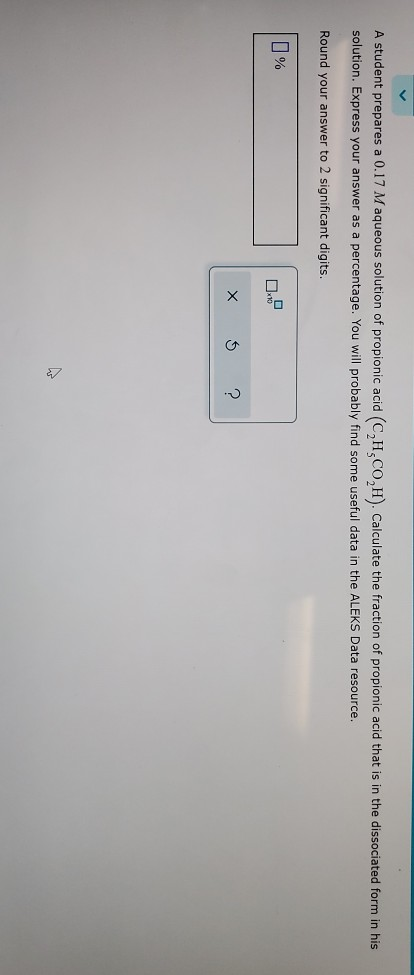
Solved A Student Prepares A 0 17 M Aqueous Solution Of Chegg Com

Solved A Student Prepares A 0 1 M Aqueous Solution Of Chegg Com

Which Of The Following 0 1 M Aqueous Solutions Will Have The Lowest Freezing Point Youtube
0 Comments